Task 00146.
Content: 00146.png (28.59 KB)
Uploaded: 05.06.2023
Positive responses: 0
Negative responses: 0
Sold: 0
Refunds: 0
$0.9
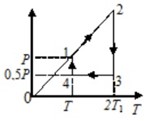
The graph shows a cycle with an ideal monatomic gas of constant mass in the amount of 2 mol. Present the graph of the cycle in P–V coordinates and determine the amount of heat received by the gas per cycle if the parameters of the gas in state 1 are T1 = 300 K and the pressure P1 = 10^5 Pa.
Detailed solution with a brief record of the conditions, formulas and laws used in the decision, the conclusion of the calculation formula and the answer.
If you have any questions about the decision, write. I will try to help. File in image format.
If you have any questions about the decision, write. I will try to help. File in image format.
No feedback yet